
#HEMERA BIOSCIENCES UPDATE#
When we hear further updates on the trials, we will update you. HMR59 is currently under FDA approved clinical testing for use in both dry and wet macular degeneration.
#HEMERA BIOSCIENCES FULL#
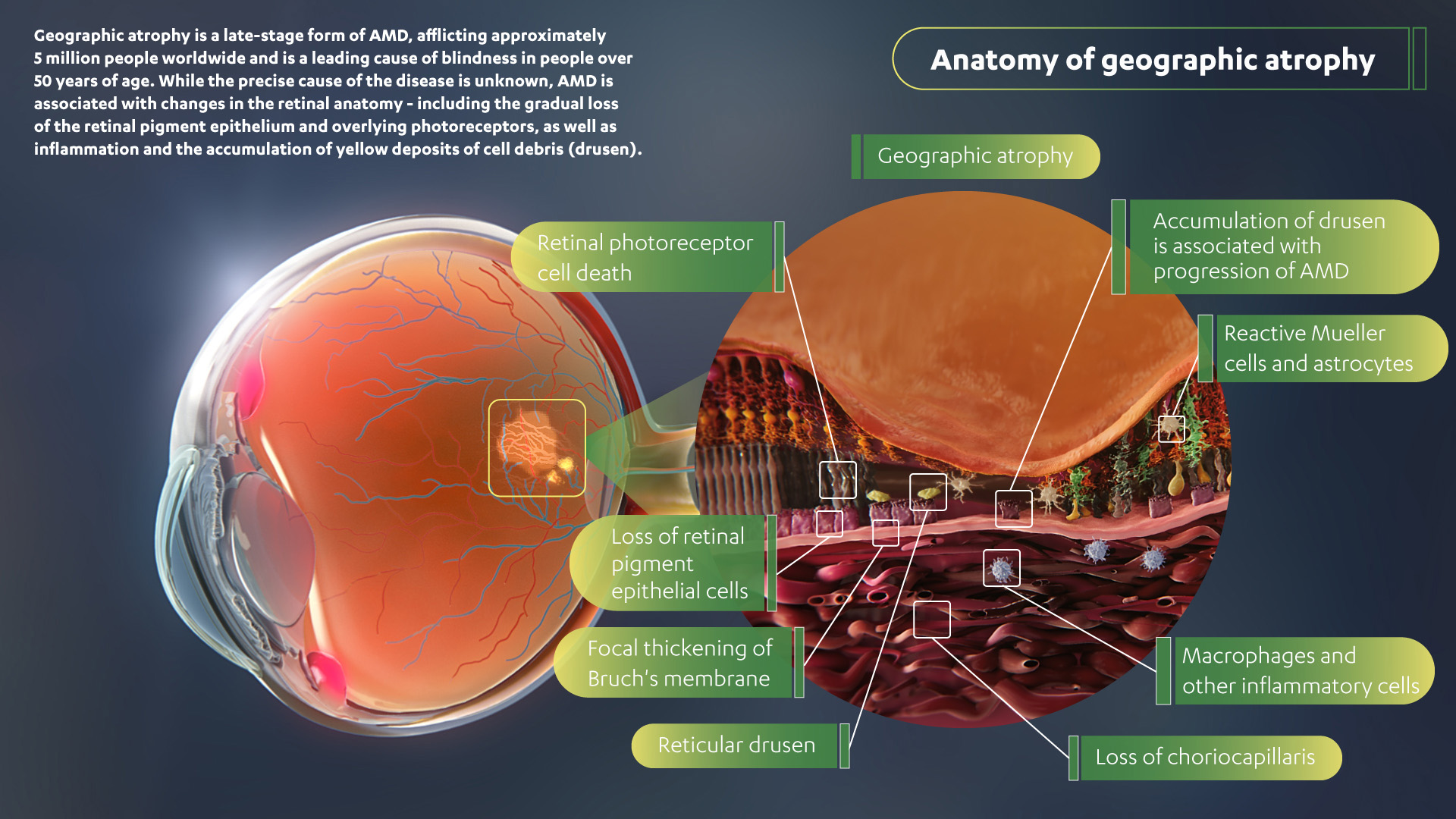
Read the full article* here Further from MDNZ This possible treatment is in the early days of research and if it becomes available in New Zealand it is likely to be at least 18 months to 2 years (it will of course also need to go through the official approval process). The company currently specializes in the Biotechnology area. for LCA2 EDIT-101 by Editas Medicine Inc, CRISPR-Cas9 for CEP290 mutations for LCA10. Systemic gene therapies typically require being processed by the liver, which becomes a much more complicated mechanism of action and delivery. Hemera Biosciences LLC is a private company that has been in the industry for 12 years. Hemera Biosciences for dry AMD 5-OPTIRPE65 by MeiraGTx UK II Ltd. It is the leading cause of blindness in people over 50 years of age.Įye diseases are something of low-hanging fruit when it comes to gene therapies, because they can be directly administered to the eye, not systemically. Geographic atrophy affects about five million people around the world. This helps prevent more damage to the retina and preserves eyesight. Annual Growth Rate (CAGR) Leading Players (Apellis Pharmaceuticals, IVERIC bio, Alkeus Pharmaceuticals, Hemera Biosciences) and Forecast to 2022-2028. The gene therapy, HMR59, increases the ability of retina cells to manufacture a soluble form of CD59. This causes a “relentless progression to blindness.” In geographic atrophy, complement overreacts and destroys cells in the macula, the central part of the retina that handles central vision and fine details. “Our aim with this novel, single-administration gene therapy is to use our development expertise and deep heritage in vision care to help improve patient outcomes by intervening early, halting the progression to blindness, and preserving more years of sight.”ĪMD patients often have low levels of CD59, a protein that protects the retina from damage caused by the body’s natural complement immune response. List, Global Therapeutic Area Head, Cardiovascular & Metabolism, Janssen Research & Development. “Geographic atrophy is a devastating form of AMD that impacts the ability to accomplish everyday tasks, such as reading, driving, cooking, or even seeing faces,” said James F. Geographic atrophy is a late-stage, severe type of age-related macular degeneration (AMD).


This therapy consists of a one-time, outpatient, intravitreal (into the eye) injection to preserve eyesight in patients with geographic atrophy (Dry MD). Janssen Pharmaceutical, a Johnson & Johnson company, has bought the rights to Hemera Biosciences’ gene therapy HMR59.


 0 kommentar(er)
0 kommentar(er)
